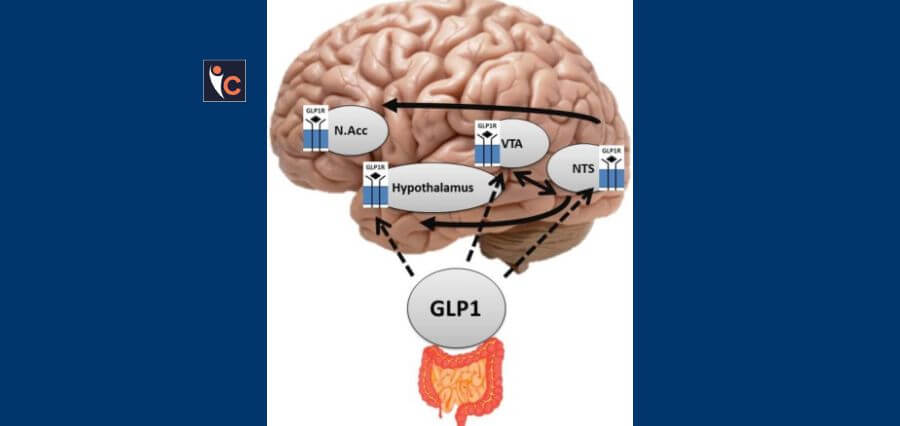
Novo Nordisk’s liraglutide, an established daily medication for diabetes and obesity marketed as Victoza and Saxenda, may have potential in slowing the progression of Alzheimer’s disease, according to data from a recent mid-stage trial. The results, presented at the Alzheimer’s Association International Conference in Philadelphia, indicate that liraglutide may offer neuroprotective benefits.
The trial, involving over 200 patients in the UK with mild to moderate Alzheimer’s, found that those administered liraglutide exhibited an 18% slower decline in cognitive function after one year compared to a placebo group. Additionally, MRI scans revealed that liraglutide significantly slowed the atrophy of brain regions critical for memory and cognitive functions by nearly 50% compared to the placebo.
Novo Nordisk’s liraglutide, primarily used for diabetes and obesity, has seen a decline in sales as patients transition to the company’s weekly injections, Ozempic and Wegovy. However, the new findings contribute to a growing body of evidence suggesting that GLP-1 receptor agonists, a class of medications that includes liraglutide, might offer benefits beyond glycemic control and weight management.
Researchers from Imperial College London, who conducted the trial with partial funding from Novo Nordisk, highlight the potential for combining GLP-1 medications with amyloid-targeting drugs, such as Kisunla and Leqembi, which have recently demonstrated efficacy in slowing Alzheimer’s progression. Notably, GLP-1s do not carry the risk of brain swelling and bleeding associated with some amyloid-targeting treatments, presenting a potential advantage for their broader use in Alzheimer’s therapy.
Dr. Paul Edison, lead author of the trial, emphasized that while further research is necessary, the promising cognitive improvements observed with liraglutide could represent a significant advancement in Alzheimer’s treatment. Novo Nordisk supports continued independent research into the potential applications of its GLP-1 products but notes that they are not currently approved for Alzheimer’s disease.
Read More: Click Here